
Time of Flight Mass Spectrometry
| Chemical Dynamics Home |
| News |
| Research Themes |
| Research Methods |
| VMI |
| LIF |
| MB |
| CMB |
| FMS |
| ToF |
| People |
| Jobs |
| Links |
| |
Last updated February 2026 |
ToFMSTime of flight mass spectrometry (ToFMS) is a mass discerning technique which when combined with a quantum state specific ionization technique, such as Resonance Enhanced Multi Photon Ionisation (REMPI), can be used to detect products from dynamical chemical processes. The classic ToFMS approach is the linear Wiley-McLaren design (Fig. 1). This design accelerates the ions using a homogeneous electrostatic field, controlled by the Repeller (R) and Ground (G) electrodes. The G electrode is formed from a fine conductive mesh, providing the homogeneous field while also allowing transmission of (the majority) of the ions. This field imparts the same kinetic energy to all of the ions. The ions then fly along a field-free ToF tube. Ions that have the same speed will arrive at the detector at the end of the flight tube at the same time. Because the same amount of kinetic energy was imparted to all ions, those with a low mass-to-charge ratio (m/z) (light molecules) will have a higher speed, and therefore will arrive first, while heavier molecules (high m/z) travel slower and arrive later. 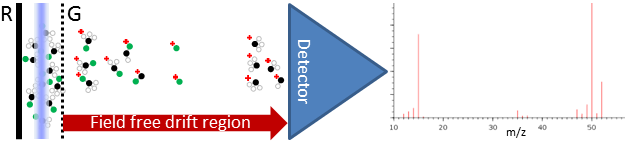
As the arrival time depends on the mass-to-charge ratio, and ions formed by REMPI are singly charged, different mass ions can be selectively detected provided the detector used has a fast (usually ns) response time. In addition to selecting the molecular species, REMPI-TOF can be used to determine information on the kinetic energy of the initial molecule. Neutral molecules that were formed flying with different velocity projections along the ToF axis will arrive at slightly different times, and provided that the ToF resolution of the spectrometer is high enough this provides a 1-d projection of the velocity distribution. This arrangement has been used quite extensively in measurements of photodissociation and reactive scattering dynamics. In 1987, Chandler and Houston introduced the technique of ion-imaging.1 They used a 2-d plate detector consisting of microchannel plates (MCPs) and a phosphor screen; when an ion strikes the MCPs a bright spot appears on the phosphor screen. They photodissociated CH3I, and after a 10 ns delay state-selectively ionised the CH3 product. The arrival positions of the CH3+ ions at the detector are a 2-d map of their 3-d spatial locations when ionised. Although a powerful technique, ion-imaging suffers from two weaknesses. One is the electrode grids used in the Wiley-Maclaren ToF configuration, which typically have no better than 50% transmission, and more significantly, introduce a grid-pattern distortion to the image. The second is the spatial mapping. Whilst this is useful in some applications, in dynamics measurements it is the velocity distribution that is required, and this is only indirectly related to the spatial distribution. Ions produced in slightly different positions in the ionisation volume are mapped to different locations, distorting the derived velocity distribution. Velocity map imaging was developed to improve upon these weaknesses, and you can read more about it here. |
|