
Molecular Beams Method
| Chemical Dynamics Home |
| News |
| Research Themes |
| Research Methods |
| VMI |
| LIF |
| MB |
| CMB |
| FMS |
| ToF |
| People |
| Jobs |
| Links |
| |
Last updated September 2025 |
MBMany of our experiments use molecular beams (MBs) to cool and control the internal and translational motions of molecules of interest. Molecular beams use the properties of an isentropic expansion from a high pressure reservoir of gas into a lower pressure region. Such an expansion may be continuous, or as in all of the examples in our labs, pulsed. A typical pulsed MB is produced using a pulsed valve, which can open a small orifice (a nozzle, typically < 1 mm diameter) for the gas to expand through in < 100 µs, hold it open for a set period of time, and then close it on a similar timescale. A typical source pressure behind the valve is in the range 1-10 bar, the vacuum chamber into which it expands will usually have a background pressure < 1 x 10-5 mbar. As the gas expands from the high-pressure zone into the low-pressure zone the atoms and molecules undergo many collisions, and the gas accelerates away from the high pressure zone. The internal degrees of freedom (e.g. rotation) are cooled, as are the relative translational degrees of freedom. Very rapidly the molecular speeds exceed the local speed of sound (Mach numer, M > 1), forming a supersonic free-jet expansion. As the expansion continues the distribution of speeds decreases, resulting in a decreasing collisional rate. Eventually collisions within the expansion come to an effective halt; the result is a pulse of gas with a supersonic mean speed, a narrow spread of speeds around that mean, with speed distribution transverse to the beam direction characterised by a temperature of a few K. Any molecules entrained in the expansion will have their rotational degrees of freedom efficiently cooled, typically to 'temperature' of a few K, although the resulting population distribution is often not well described by a Boltzmann function. Around the sides of the expansion the cooling will be less efficient, ultimately reaching a shock boundary where the speed drops below supersonic. It is therefore usual to place a second aperture, called a 'skimmer' downstream from the nozzle along the centreline of the expansion (see Fig. 1). The skimmer 'cores' the expansion, selecting only the coldest and densest central portion of the expansion. Conventionally the skimmer is placed on a vacuum bulkhead separating a source MB chamber from the experimental chamber in which the MB will be used, secondary collimation of the MB within subsequent chambers is also sometimes applied. The final speed of the MB is determined by the thermodynamics of the expansion, depending on the temperature of the source, the heat capacity of the gas, and the molecular mass of the gas. MBs of the noble gases (He, Ne, Ar, Kr and Xe) are the easiest to produce, and provide a range of beam speeds. MBs of the rare gases seeded with small fractions (typcially < 10%) of molecular species have properties very similar to the beams of pure noble gas, and provide a way to cool the internal degrees of freedom of molecules and control their translational energy. The cooling and control that MBs provide sees them used in a wide range of experiments. Molecular spectroscopy benefits in resolution from the reduction of the Doppler effect resulting from the narrow spread of transverse speeds, as well as the simplification of spectra arising from the small number of populated states. Photodissociation dynamics benefits from the well defined and narrow range of laboratory speeds, reducing the blurring effects that motion of the precursor introduces, and narrowing the distribtion of internal energies. Finally, the well defined speed and angular spread of MBs makes them the ideal starting point for collisional studies, either gas-surface, or when two MBs are combined in crossed molecular beams (CMBs), gas-phase bimolecular inelastic or reactive scattering. 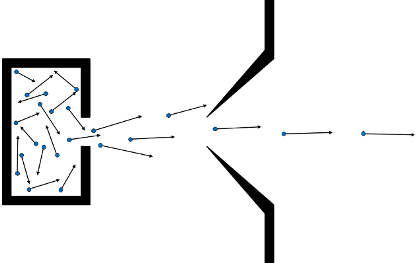
|
Direct Current Discharge Radical Production
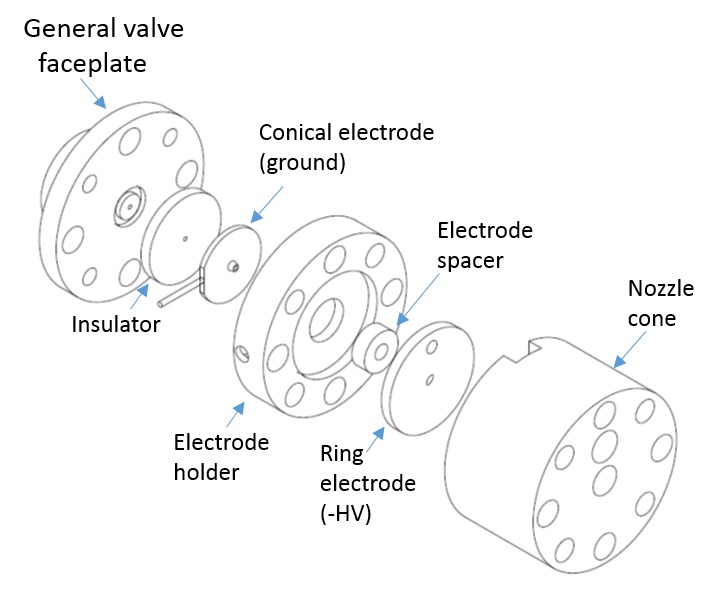
Unstable radical species, e.g. OH, may be produced in a molecular beam by electric discharge induced breakdown of a suitable precursor. We have succesfully implemented this approach to generate OH from H2O seeded in He. The source consists of two circular electrodes separated by an insulating spacer, which fit onto (and are insulated from) the faceplate of a Parker "General" solenoid valve. The electrodes have a 1 mm diameter, matching the faceplace aperture. The electrode nearest the faceplate has a conical form, and is held at ground. The second electrode has a ring shape, and a large negative voltage (e.g. - 1000 V) is applied to this to initiate the discharge. The gas then expands through a nozzle cone, resulting in a supersonic beam containing radical products from the breakdown of the seed gas.
|
|
Fig 2. Exploded diagram of the discharge source |
Rapidly pulsing the applied HV (e.g. a 10 μs square HV pulse) results in the generation of a short packet of radicals embedded in the longer (typically 200 μs) molecular beam pulse. This has provided a suitable starting point for our studies of OH inelastic scattering at gas-liquid interfaces.