
Oxygen Radical Dynamics
The study of reactions at the gas-liquid interface presents a challenge due to the complex nature of liquid surfaces. We have developed an experimental method in which we detect the gas-phase products spectroscopically after reaction/collision with a liquid surface, deriving internal and translational energy distributions. This allows us to interrogate the fundamental steps involved in gas-liquid interfacial processes.
Inelastic Scattering of OH(D) at Liquid Surfaces
Inelastic Scattering of OH(D) at Liquid Surfaces
OH radicals present in the troposphere are the predominant daytime atmospheric oxidants. The reaction of OH with organic molecules in aerosols has an impact on our climate; therefore it is important to gain an understanding of its mechanism. In our group we study these processes from a fundamental point of view, characterizing the collision dynamics of OH radicals with different hydrocarbon liquids. OH is generated by photolysis of a suitable precursor, and radicals that scatter from the surface are detected by LIF. We investigate scattering of OH molecules from reactive and inert liquid surfaces. This gives us the opportunity to estimate the fraction of OH radicals which do not escape from liquid surfaces. Potentially reactive hydrocarbons with abstractable H atoms (squalane, squalene and oleic acid) produce less scattered OH than the inert liquid PFPE. A comparison between squalane and squalene provides evidence that the thermalised products are lost on addition to the unsaturated sites (see figure below). Squalane is the most efficient at energy transfer to the surface, whereas PFPE gives a more elastic surface resulting in scattered products with higher energy. Using a different OH precursor (allyl alcohol or HONO) produces incoming OH radicals with different energy distributions, providing complementary information on the scattering dynamics. Rotationally cold OH radicals from HONO are suitable for observing translational-to-rotational energy transfer in direct collisions. In contrast, the broader OH rotational distributions obtained from allyl alcohol allow us to distinguish between directly scattered and thermalised products.
|
|
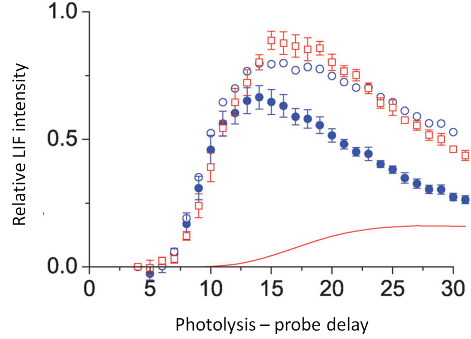
OH appearance profiles from squalane (red open squares) and squalene (blue filled circles), acquired in the Q1(1) transition of the A-X (1,0) band by varying the delay between photolysis and probe laser pulses. Composite profiles (blue open circles) are the sum of the squalene profile and an adjustable weighted contribution from a Monte-Carlo simulation of a thermal component (solid red line). |
Control of the collision energy, and reduction in this towards energies more applicable to collisions in the Earth's atmosphere, has been achieved through the implementation of an OH-containing molecular beam, using a HV DC pulsed-discharge in a rare gas/water mixture. This results in a 'packet' of OH with a well-defined collision energy, and cold (30-40 K) rotational distribution that may be directed at the gas-liquid surface. LIF probing of the scattered products has been applied in the same fashion as in our previous photolytically initiated experiments. Repeating these experiments with the carrier gases He (OH collision energy 29 kJ mol-1) and Ne (7.5 kJ mol-1) has provided new insight into the collision energy dependence of OH uptake at squalane and squalene surfaces. Uptake at squalene surfaces is found to be negatively activated (i.e. faster at lower collision energies). This is consistent with an increasing contribution from addition to the unsaturated double bonds in squalene as the collision energy is reduced, which is a barrierless process. Surprisingly, uptake at the squalane surface is found to be more or less independent of collision energy. This is surprising, as analysis based on the known bond activation energies for H-abstraction by OH predicts a significant reduction in reactive uptake at the lower, Ne carrier gas, collision energy. Our current hypothesis is that this insensitivity to collision energy reflects the balance between impulsive scattering and surface accomodation of OH at the squalane surface changing with collision energy. At lower collision energy, fewer direct impulsive collision result in reaction, but a larger fraction of the overall collisions result in accomodation at the surface, allowing multiple surface interactions with a resulting increase in abstraction probability, despite the lower energy.
|
|
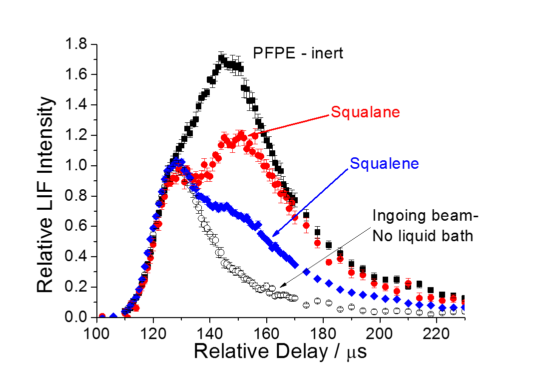
OH appearance profiles from ingoing MB (black open), and the surfaces: PFPE (black filled), squalane (red) and squalene (blue), acquired on the Q1(5) transition of the A-X (1,0) band by varying the delay between HV discharge and probe laser pulses. |
Real-Space LIF Imaging of Surface Scattering
We have recently, for the first time, demonstrated real-space laser-induced fluorescence imaging of OD radicals scattering from the liquid surface. The packet of OD radicals produced by the discharge molecular beam source is directed at the liquid surface as in our previous experiments. However, the probe laser beam has now been expanded into a horizontal sheet of light, which runs parallel to the surface and contains the in-going beam and the surface normal. An imaging assembly of lenses and a band-pass filter collects the fluorescence from the OD radicals and images their spatial locations onto an in-vacuum image intensifier. This amplifies the weak fluorescence (and is capable of detecting single photons) and turns them into a bright visible light image on a phosphor screen that is captured by a conventional scientific CCD camera. By varying the delay between the creation of the OD packet in the discharge and the probe laser pulse, we can create movies of OD packet (for a specified rotational level) travelling in towards the surface, and then the 'wave' of inelastically scattered OD recoiling back into vacuum.
|
|
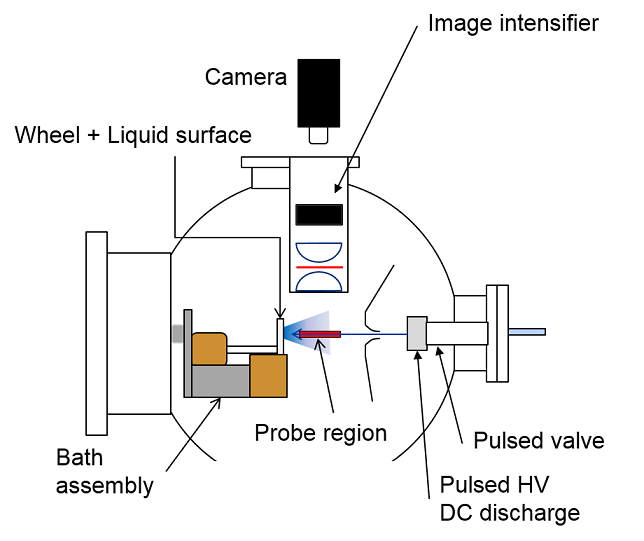
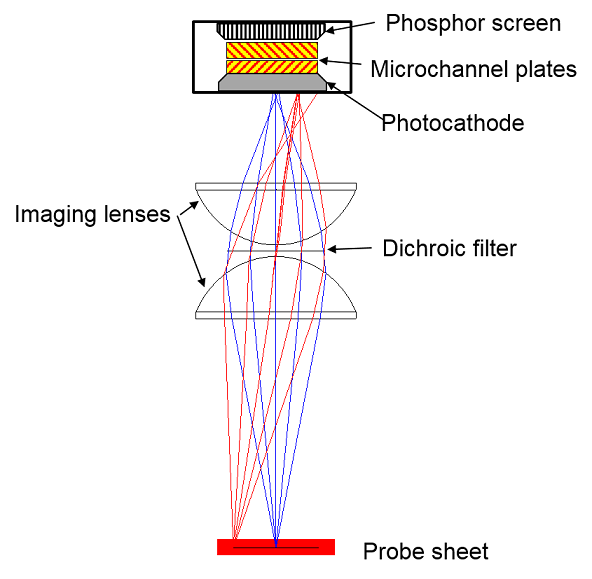
(Left) The scattering apparatus, showing the arrangement of the liquid coated wheels, pulsed molecular beam source and real-space LIF imaging. (Right) A close-up of the imaging system, showing the lenses, filter and the components of the image intensifier. |
We have used this apparatus to make the first measurements of rotational state resolved stereodynamics for inelastic scattering of OD at inert (PFPE) and reactive (squalane, squalene) liquid surfaces.

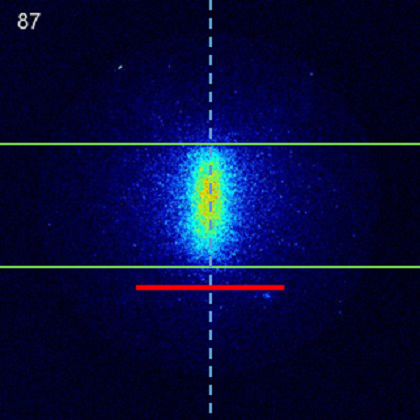

|
|
Movies of OD N = 6 scattering from a PFPE liquid surface. The static image shows a frame containing the in-going molecular beam packet, with the green lines indicating the limits of the probe laser sheet, the red line denoting the location of the liquid surface, and the dotted line the surface normal. The number in the top left corner of each frame is the time in microseconds since the triggering of the pulsed discharge that creates the OD packet. The movie on the left initially shows an in-going packet proceeding at normal incidence towards the surface, with an average velocity of 1800 ms-1. The packet is quite tightly focussed in the horizontal direction, a result of the skimming of the molecular beam, and is about the length of the probe laser sheet (approx 30 mm). It disappears out of view at the bottom of the laser sheet briefly, and is replace by a broad out-going scattered wave. In this case, the peak intensities of the in-going beam and scattered wave are approximately equal, however, note that the overwhelming majority of the in-going OD is in lower initial rotational states, and the majority of the scattered wave is the result of rotational excitation at the surface, i.e. translational to rotational energy transfer. The scattered plume is relatively broad, and is symmetric about the surface normal, as required by the symmetry of the scattering system. The right hand movie shows the identical scattering process, but this time for an incident angle of 45 degrees. It is immediately apparent that the angular distribution of the scattered products is very broad and very nearly symmeteric about the surface normal. There is no clear evidence of 'specular' scattering. This implies that the liquid surface is 'rough' on a molecular scale, and that scattering is almost equally likely into a wide range of final angles. A more quantitative analysis of the scattered OD yields information about the angular scattering, and also about the speed distribution as a function of incident and final angle, for individual product rotational states. We observe superthermal scattered OD speeds, which depend on incident and final angle, and provide clear evidence of an impulsive scattering process, in which the OD undergoes only a few interactions with the surface and does not spend sufficient time at the surface to equilibrate. |
Reactive scattering of O(3P) + hydrocarbons
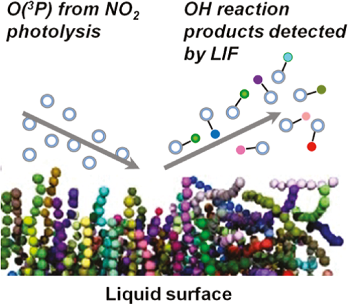
In these experiments, gaseous O(3P) atoms are created by laser photolysis of NO2 at a short distance above the liquid surface. On impact with the surface, some of the O(3P) abstracts a hydrogen atom, generating OH radicals that escape from the surface. These are probed in the gas phase by laser induced fluorescence (LIF).
|
We have successfully employed this method to study interfacial reactions with a variety of hydrocarbons and self-assembled monolayers. Our most recent work focused on introducing unsaturated sites in the hydrocarbon backbone to study the effect on the interfacial dynamics. The partially unsaturated hydrocarbon squalene was compared against its saturated analogue, squalane. The scattered products show different energy distributions due to inherently different H-abstraction dynamics, but there is also partial thermal accommodation of OH at the liquid surface, a feature of most gas-liquid reactions. The OH yields from both liquids are dramatically different, indicating the presence of unsaturated groups at the squalene surface. The O(3P) atoms are likely to be lost by competitive addition to the double bonds.
|
|
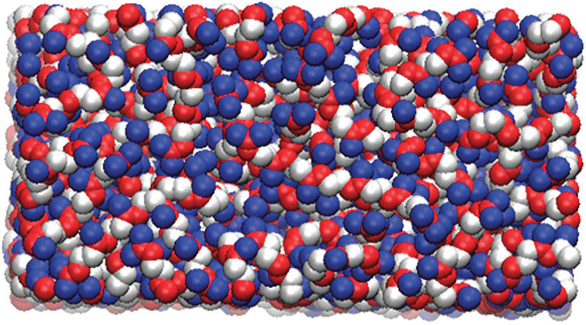
MD snapshot of a squalene surface (top view). Red atoms indicate unsaturated sites. |
-
Real-space laser-induced fluorescence imaging applied to gas-liquid interfacial scattering
Journal of Chemical Physics (2019) 151, 054201
doi: 10.1063/1.5110517
-
Collision-Energy Dependence of the Uptake of Hydroxyl Radicals at Atmospherically Relevant Liquid Surfaces
Journal of Physical Chemistry C (2018) 122, 6648
-
Site and bond-specific dynamics of reactions at the gas-liquid interface
Physical Chemistry Chemical Physics (2014) 16, 173
doi: 10.1039/c3cp54107j
Collision Dynamics and reactive uptake of OH radicals at liquid surfaces of atmospheric interest
Physical Chemistry Chemical Physics (2011), 13, 8457
doi: 10.1039/c0cp02734k
Dynamics of the Gas-Liquid Interfacial Reaction of O(1D) with a Liquid Hydrocarbon
Journal of Physical Chemistry A (2011), 115, 7210
doi: doi
Go to top