
CN & HCN Dynamics
Reactive Scattering Dynamics at the Gas-Liquid Interface: Bridging the Gap between the Gas-Phase and Solution
This EPSRC-funded project combines our prior knowledge of the inelastic and reactive scattering at liquid surfaces with gas-phase detection via Frequency Modulated Spectroscopy (FMS). In addition, state-of the-art molecular dynamics simulations, carried out in the group of Dr. David Glowacki (University of Bristol), will provide microscopic insight into the interactions and reactions at the gas-liquid interface.
Anywhere the gas and liquid phases meet, chemistry occurs at the interface. Examples in the natural world include: respiration in living organisms; atmospheric aerosol particles; the surface of the sea on Earth; hydrocarbon particles in the atmosphere of Saturn's moon Titan. The chemistry of these interfaces is also vital in man-made environments as well: combustion of liquid fuels; industrial processes such as multiphase catalysis, gas sequestration and distillation. However, despite their importance, in comparison to the chemistry of reactions in the gas-phase or in solution, reactions at the gas-liquid interface are much less well understood. This project aims to deepen our fundamental understanding of reactions at liquid surfaces through a combination of cutting-edge experiment and theory.
Consider a gas molecule approaching a liquid surface. The first encounter it makes with the surface will be an isolated event; the gas phase molecule will collide with a single molecule of the liquid surface. At this point the encounter is essentially the same as a gas phase collision between two isolated molecules. In the gas-phase, the molecules will then recoil and the encounter will be over. In some cases, collisions at the liquid surface will also result in the gas-phase molecule rebounding back into the gas-phase. However, it may instead go on to collide with further liquid surface molecules, and may even pass through the surface of the liquid and into solution, before eventually returning to the gas-phase. Reactions at the gas-liquid interface thus share characteristics of both the gas and solution phases, and by studying the dynamics of the reactions we can bridge the gap between them. This complements the intensive on-going effort in these hitherto largely separate areas, providing a unifying picture of molecular scattering dynamics.
We have developed a new apparatus for our experiments, based on our previous experience in gas-liquid interfacial scattering, and have combined it with high-resolution laser spectroscopy previously applied to study gas-phase dynamics. We use this to study the reaction of CN radicals with liquid hydrocarbons, which forms HCN. The dynamics of this benchmark reaction process have been previously studied in the gas and solution phases. This reaction is not only of fundamental interest, as the CN radical is an important reactive species in extra-terrestrial atmospheres (e.g. atmosphere of Titan), and liquid hydrocarbon combustion. Simultaneously with the experiments, we will develop new theoretical models of the forces between the atoms present, and use those in calculations to simulate the dynamics of the reactions under experimental conditions. We will compare and combine the results of the experiments and theory to provide the most-detailed ever description of gas-liquid interfacial reaction dynamics. The fundamental insights into dynamics at the gas-liquid interface provided by this work will inform our understanding and modelling of the processes at gas-liquid interfaces in a wide range of environments vital to our society, e.g. atmospheric aerosols, liquid fuel combustion.
Experimental Layout
|
The experimental apparatus at Heriot-Watt consists of two differentially pumped stainless steel high vacuum chambers. The ‘source’ chamber houses a pulsed solenoid valve which generates a molecular beam via supersonic expansion. The molecular beam is composed of BrCN seeded in a rare gas carrier. A pulsed electric discharge at the throat of the valve initiates the dissociation of BrCN generating CN radicals. The molecular beam then passed through a skimmer before entering the ‘main’ chamber. The main chamber houses a stainless steel wheel which is partially submerged in a low vapour pressure liquid. Rotating this wheel therefore provides a continually refreshed surface to scatter the incoming molecular beam. The ingoing molecular beam and scattered products are detected in the gas-phase upon passing through a multi-pass optical cell which is adjacent to the liquid surface. Information obtained about the rotational state distribution, time-of-flight and Doppler shift gives insights into the dynamics that are occurring. |
Fig.1. A Virtual Tour of the Experimental Apparatus. |
Inelastic scattering of CN + hydrocarbons
|
Fig.2. A simulation of the interaction of an CN radical with a gas-phase squalane molecule performed by our collaborators at The University of Bristol. |
In the first experiments the CN radicals will be scattering off the surface of an inert liquid such as PFPE. The ingoing and scattered CN radicals can be probed using FMS on the Near Infrared (NIR) A-X (2,0) band as the radicals passes through the multipass optical cell. In later experiments, the CN radicals will be scattered from the surface of reactive hydrocarbons and comparisons with PFPE will provide information about the relative survival probabilities of the CN radicals. The elastic scattering of a CN radical off a gas-phase hydrocarbon can be seen in figure 2. |
Reactive scattering of CN and Inelastic scattering of HCN
|
In subsequent experiments the reactive pathway, in which the CN radicals abstract a hydrogen atom from the hydrocarbon surface, will be studied. To probe the product, HCN, a Mid Infrared (MIR) source is required. This modulated MIR source is achieved by integrating a modulated Continuous Wave (cw) Ti:Sapphire Laser (750-950 nm) with a cw Fibre laser (1064 nm) to perform Difference Frequency Generation (DFG) in a crystal of Periodically Poled Lithium Niobate (PPLN). Hence, generating a tuneable narrow-band 3 μm source suitable for HCN absorption spectroscopy. |
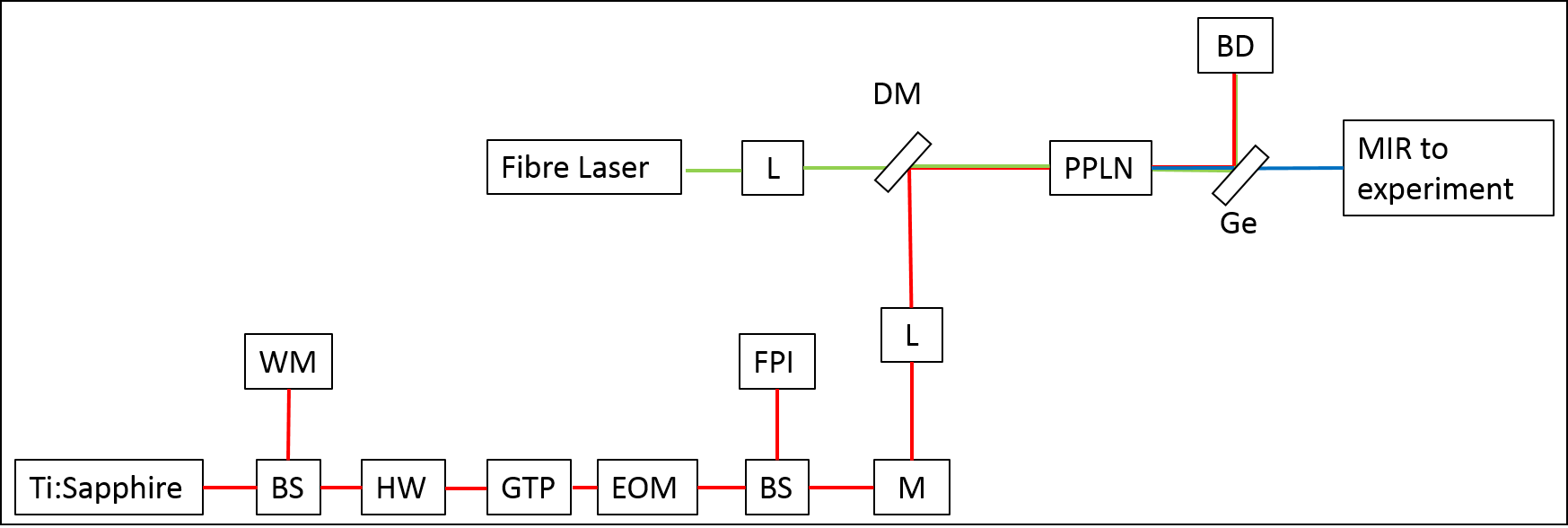
Fig 3. A Schematic diagram of the Mid-IR source. BS, Beam Splitter; WM, Wavemeter; HW, Achromatic Half-Wave Plate; GTP, Glan-Taylor Polariser; EOM, Electro-Optic Modulator; FPI, Fabry-Perot Interferometer; M, Mirror; L, Lense; DM, Dichroic Mirror; PPLN, Periodically Poled Lithium Niobate; Ge, Germanium Filter; BD, Beam Dump. |
Go to top