Analytical Applications
Ionic liquids (ILs) are salts that are molten below 100 °C. They can display a wide range of physicochemical properties, which makes them potentially suitable for a great variety of applications such as fuel cells, gas detection and catalysis, and especially as green solvents in chemical synthesis. Many of these applications involve an interaction between the ionic liquid surface and molecules in the gas phase, so the surface composition plays a key role in the process.
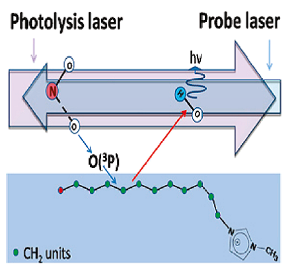
In this experiment we use reactive scattering of ground-state O(3P) atoms to investigate the surface structure of ionic liquids. The aim is to determine whether the surface composition is different from that of the bulk, and how the nature of the ions influences their arrangement at the interface. The O(3P) atoms react selectively with hydrocarbon chains in the liquid and produce OH radicals. OH products scattered back into the gas phase are then detected by laser-induced fluorescence (LIF). Since O(3P) reacts selectively with the -CH2- groups in the IL, the interfacial reactivity provides a measure of the extent of surface occupation by alkyl chains.
|
|
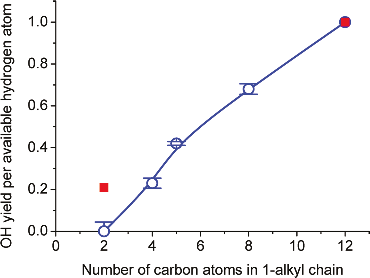
This method has been applied with a family of 1-alkyl-3-methylimidazolium ionic liquids, [Cnmim][NTf2] (See references below as well as our Perspective Video). The work was carried out in collaboration with the groups of John Slattery (University of York), Tim Minton (Montana State University) and George Schatz (Northwestern University). It was shown that the reactivity of the ionic liquids increases with the alkyl chain length in a way that cannot be explained solely by stoichiometry. Plotted below is the relative reactivity of the ionic liquids studied with respect to the number of CH2 units in their alkyl chain. In absence of surface segregation, we would expect this normalised yield to be constant. In contrast there is a sharp increase which gives evidence of a surface enrichment of alkyl chains as n, the number of carbon atoms in the alkyl chain, increases. Complementary experiments at higher collision energies and molecular dynamics simulations were in agreement with this result.
|
|
Relative OH yield per available hydrogen atom as a function of alkyl chain length for a series of [Cnmim][NTf2] ionic liquids (blue open circles). n is the number of carbon atoms in the cation alkyl chain and [NTf2] stands for bis(trifluoromethylsulfonyl)imide, the anion in all studied ILs. |
Ionic Liquid-Vacuum Interfaces Probed by Reactive Atom Scattering:Influence of Alkyl Chain Length and Anion Volume
Journal of Physical Chemistry C (2015), 119, 5491
doi: 10.1021/jp512638
Reactive Scattering as a Chemically Specific Analytical Probe of Liquid Surfaces
Journal of Physical Chemistry Letters (2011), 2, 12
doi: 10.1021/jz1013032
O(3P) Atoms as a Probe of Surface Ordering in 1-Alkyl-3-methylimidazolium-Based Ionic Liquids
Journal of Physical Chemistry Letters (2010), 1, 429
doi: 10.1021/jz900247y
O(3P) Atoms as a Chemical Probe of Surface Ordering in Ionic Liquids
Journal of Physical Chemistry A (2010), 114, 4896
doi: 10.1021/jp912045j
Go to top