
Laser Induced Fluorescence Method
| Chemical Dynamics Home |
| News |
| Research Themes |
| Research Methods |
| VMI |
| LIF |
| MB |
| CMB |
| FMS |
| ToF |
| People |
| Jobs |
| Links |
| |
Last updated September 2025 |
LIFLaser-induced fluorescence (LIF) is a widely used spectroscopic technique first developed by Zare and co-workers.1 In a LIF experiment; a laser excites a chemical species from a specific rotational-vibrational level in its ground state to a rotational-vibrational level in the excited electronic state. The excited species then undergoes a spontaneous transition back to the ground electronic state, with the product states determined by the rotational selection rules and vibrational Franck-Condon overlap. The emitted photons are then detected with a sensitive light detector, usually a photomultiplier tube. The wavelength (λ) of the excitation light can be varied if a tuneable laser is used. As the excitation wavelength changes, the atoms or molecules are excited and subsequently fluoresce whenever λ is resonant with a specific rovibronic transition, resulting in an excitation spectrum. Each transition wavelength detects a specific rovibronic level in the lower electronic state, and the intensity of the fluorescence is proportional to the number density of molecules in that state. Whilst there are many factors that determine the absolute signal size in LIF, it is generally straightforward to convert the measured signal sizes to relative populations for different rotational states, and with somewhat greater difficulty, relative vibrational populations may be determined. 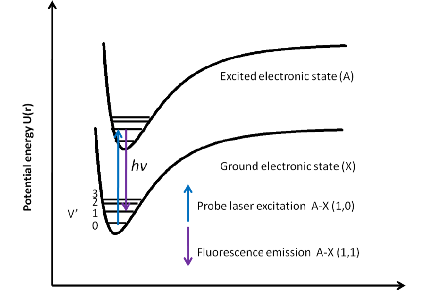
In order to be successfully studied by LIF, a species must have a known spectrum, a strong electronic absorption band within the probe wavelength range, and good fluorescence quantum yields with no predissociation in the excited state. A vast range of molecules, atoms and radicals satisfy these conditions, which makes LIF suitable for many applications. One of the major advantages of LIF over absorption spectroscopy is its sensitivity: it is a zero-background technique with excellent signal-to-noise ratios. For example, it can be used to detect low concentrations of radical intermediates in flames and plasmas. LIF is also a particularly useful tool for the study of gas-phase collision dynamics, as it provides the internal energy distribution of the products. In addition, the polarisation of the light gives information about the orientation and alignment of the collision products. |
|