
O(3P) + D2 → OD(X2∏) + D
The dynamics of the reaction of O(3P) with molecular hydrogen are being studied in collaboration with Tim Minton's group at Montana State University
|
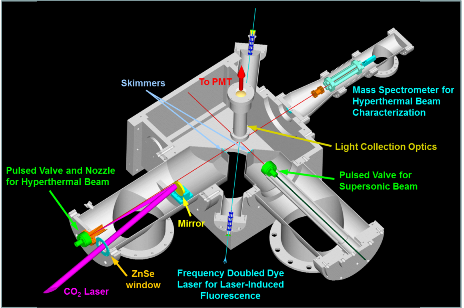
We have used a unique laser-detonation source at MSU to generate high-energy atoms, combined with laser-induced fluorescence (LIF) detection of the nascent OD products. We have, for the first time, been able to successfully determine product-state distributions fundamentally and practically important three-atom system. We use D2 rather than H2 because it has kinematic advantages in overcoming the intrinsically high barrier, which has been the main hurdle preventing previous studies of the dynamics of this system.
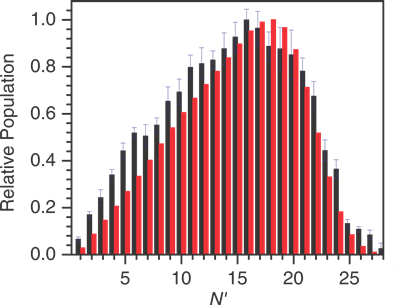
We have characterised the branching over OD product states, including not only the mechanical rotational and vibrational motions but also the fine-structure (spin-orbit) and parity (Λ-doublet) states. The rovibrational distributions are in good agreement with prior theory by George Schatz's group on the accepted potential energy surfaces.
|
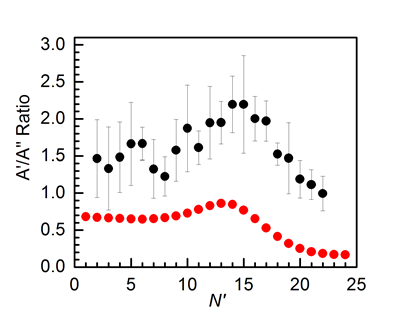
However, we find several surprises in the branching over the electronic fine-structure states. There is no preference experimentally for either of the OD spin-orbit states, despite repeated predictions that the lower, F1 (or 2∏3/2), state should be favoured based on the accepted, reduced dimensional four-state theoretical model of Hoffmann and Schatz. Conversely, although there have been no rigorous theoretical predictions of the L-doublet ratio, we find a clear preference for the ∏(A') levels. This contradicts a simple extrapolation of the ratio of the calculated cross sections on the 3A' and 3A" potential energy surfaces which, in a sudden limit, would also correspond to the OD product Λ-doublet ratio, but favour the opposite, ∏(A"), levels.
Our work has stimulated theoretical interest (see Millard Alexander's Nature Chemistry commentary) and we hope that it will lead to more theoretical effort on non-adiabatic electronic effects in this benchmark elementary system, which is among the simplest for which spin-orbit coupling extends from reactants through intermediate geometries to the products.
|
Product-State-Resolved Dynamics of the Elementary Reaction of Atomic Oxygen with Molecular Hydrogen, O(3P) + D2 → OD(X2∏) + De
Nature Chemistry (2013), 5, 315
doi: 10.1038/NCHEM.158
Go to top